The development of new cancer drugs is one of the greatest challenges in modern medicine. A key reason lies in the models used during the preclinical phase, before testing in humans. Here, animal models and cell-based tests are employed to predict the efficacy and safety of a drug. However, these systems have limitations: They frequently fail to adequately replicate the complex biological processes in human tumors and metastases. As a result, many substances that appear promising in the preclinical phase are later found to be ineffective or unsafe in clinical trials. This leads to enormous costs, delayed progress, and missed opportunities to make life-saving therapies available to patients in a timely manner.
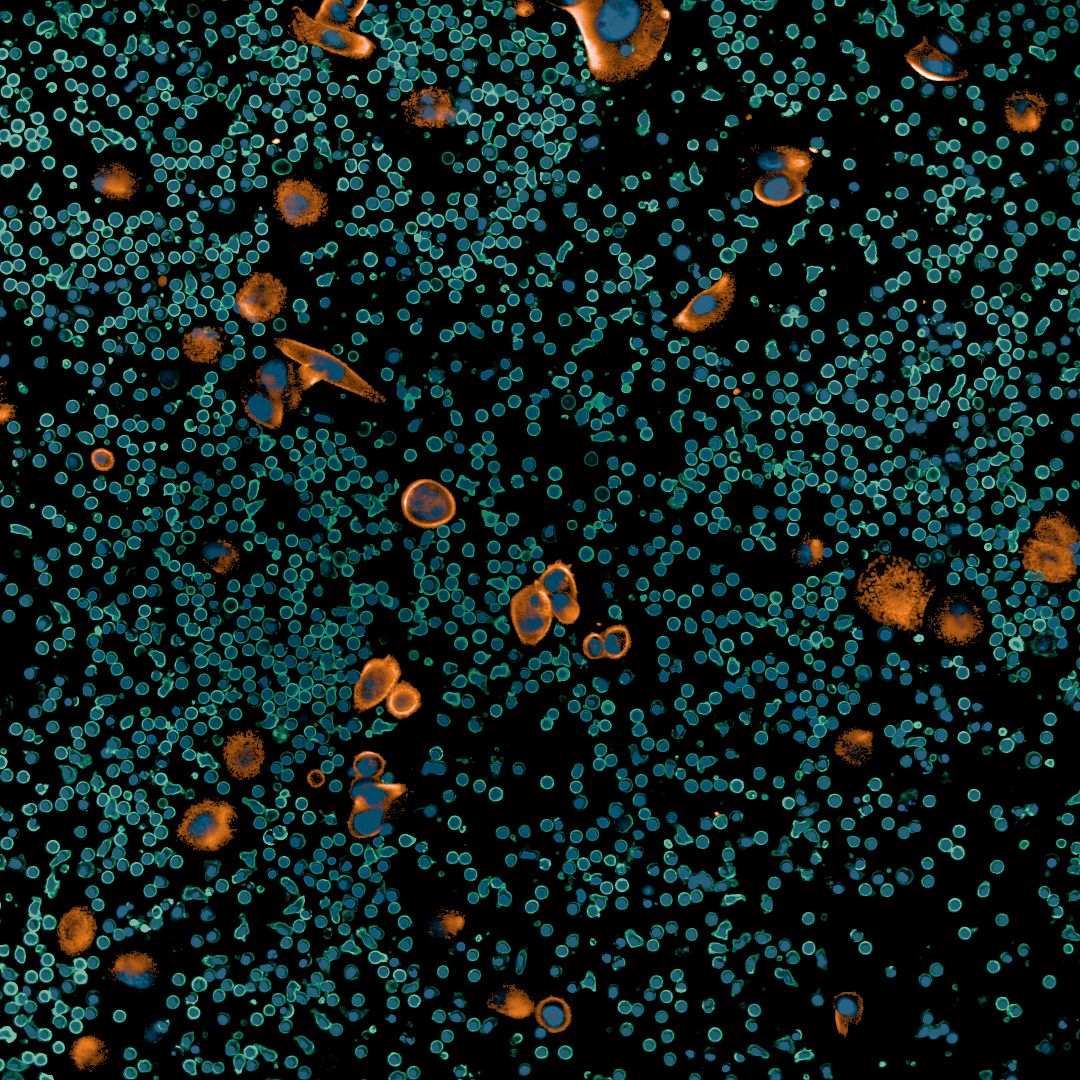
Scientists at Fraunhofer ITEM in Regensburg have developed a promising approach to overcome these hurdles. With the PEDRA platform (Patient-Derived Ex-Vivo Drug Response Assays), drugs can be tested directly on primary tumor samples from cancer patients. The patients' cells are briefly cultured in their original composition, treated with relevant drugs, and then analyzed using high-throughput microscopy. Using advanced image analysis algorithms, the effects of the drugs on both tumor and immune cells can be determined within just a few days.
In initial studies, PEDRA has already demonstrated its ability to correctly identify drug resistance and suggest experimental therapies. Currently, the platform’s potential to predict the efficacy and safety of new cancer drugs is being thoroughly investigated in collaboration with the Bavarian Cancer Research Center (BZKF). Moreover, studies funded by the Bavarian State Ministry and the German Federal Ministry of Education and Research (BMBF) are examining how PEDRA can be used to personalize cancer drug predictions for individual patients. By doing so, PEDRA could not only accelerate drug development but also help to ensure that patients benefit from tailored and more effective therapies.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine
