Applying stem cell technologies and differentiated cell types for safety and efficacy testing
In recent years, stem cell technologies have revolutionized biomedical research, leading to major advances in our understanding of diseases, the fundamental biology of the human body, and the development of novel therapeutic approaches. The great potential of pluripotent stem cells lies in their ability to differentiate into virtually any cell type of the human body while maintaining unlimited self-renewal capacity.
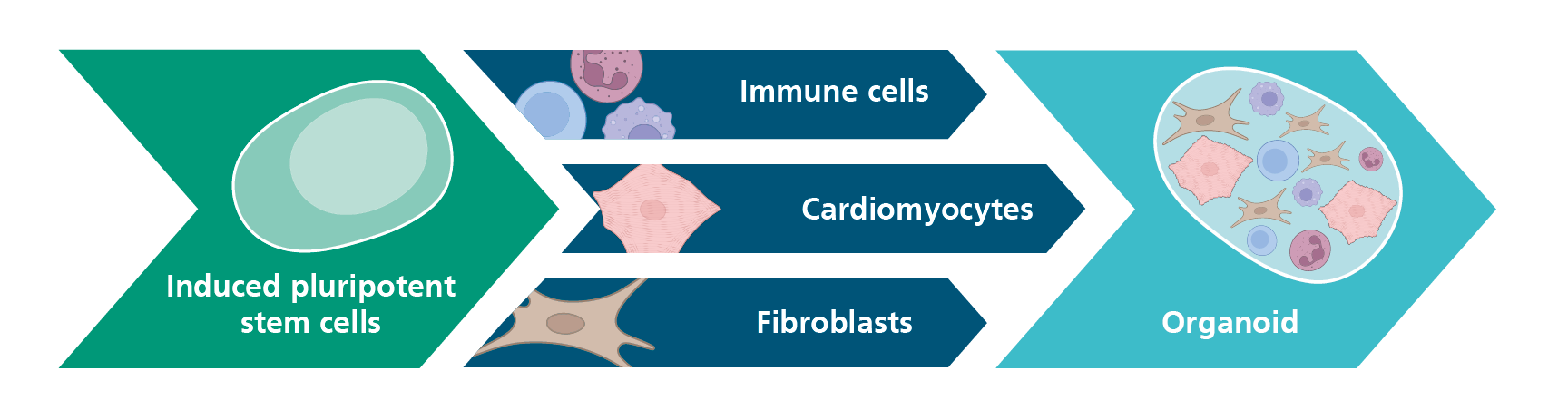
At Fraunhofer ITEM, we use induced pluripotent stem cell (iPSC) technologies for a variety of applications and differentiate these stem cells into different cell types. Our current focus is on human immune cells and cardiac cell types – particularly cardiomyocytes – which can be used, for example, in toxicity testing and immunoassays. This unique expertise also forms the foundation for the development of 3D organoid systems.
We are committed to providing standardized, quality-assured, and human-relevant cell systems and innovative models for pharmaceutical research and toxicity assessment.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine