Scientific studies on the reliability and relevance of new approach methodologies (NAMs) as alternatives to animal studies
Research on alternatives to animal studies on behalf of European Chemicals Agency (ECHA)
The European Chemicals Agency (ECHA) has contracted a consortium led by the Fraunhofer Institute for Toxicology and Experimental Medicine ITEM, coordinated together with Michabo Health Science and BASF Metabolome Solutions, to conduct scientific studies on the reliability and relevance of new approach methodologies (NAMs) as alternatives to animal studies and to promote the use of such methods in the future. The aim of the contract is to get additional NAMs accepted by regulatory authorities, focusing on OMICS technologies and toxicokinetics, and therewith further reduce the number of animal studies conducted as part of safety assessments for chemical substances. The contract will run for six years for a total value of 4.2 million euros in ECHA funding. Other partners include the department for experimental toxicology and ecology at BASF, the University of Birmingham, and the biotech companies BioClavis, and Novogene Europe.
Toxicologist Dr. Sylvia Escher, Head of Department In-silico Toxicology at Fraunhofer ITEM emphazises: “This project will promote the integration of NAMs such as multi-omic and physiologically based kinetic modelling into hazard assessment, so that eventually animal testing wouldn’t be necessary any longer for every single substance.”
The research partners will support ECHA on developing guidelines that can be used to reliably predict the properties of substances for which there is not yet sufficient safety information. The “grouping and read-across” approach uses existing toxicity data from structurally similar substances to make these predictions - a method that is already being applied to fill data gaps in registrations under the REACH regulation (EU regulation on the registration, evaluation, authorization and restriction of chemicals).
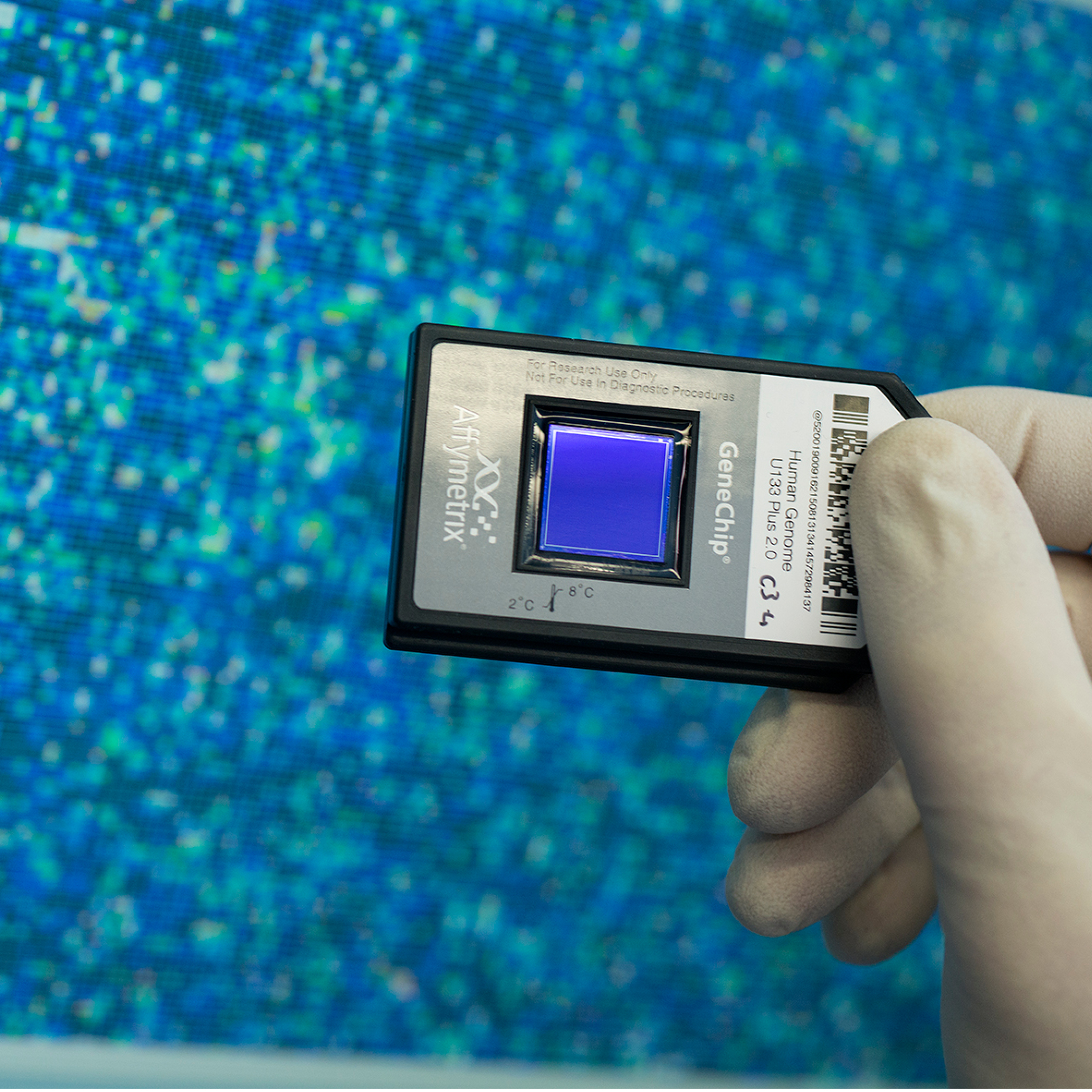
A primary focus of the research is evaluating the informative value of OMICS technologies in chemical safety assessments. These technologies can be used to study, among other things, the activation of genes (transcriptomics) or entire metabolic processes (metabolomics) in cultured cells or a living organism.
With OMICS technologies, researchers can measure numerous different changes in a biological sample to extrapolate whether substances have a potentially hazardous effect. Transcriptomics can, for example, determine how the activities of genes change after exposure to a certain substance. The researchers can then draw conclusions about changes to cells or organs. Metabolomics technologies can be used to study metabolic products in cells, such as amino acids, lipids or hormones. If these change, the health of an organism can be evaluated, much like a diagnostic blood test at the doctor’s office.
The research team aims to find out under which conditions the OMICS technologies can reliably deliver relevant and reproducible findings in order to assess the safety of chemical substances. Regulatory authorities should then be able to consult these findings when assessing the substances. This could also further reduce the number of animal studies needed in the future.
The project will also evaluate methods to reliably predict the kinetic behaviour of substances in an organism, including how they are absorbed, distributed and excreted as well as to evaluate the accumulation and degradation of substances. To facilitate the application and implementation of physiologically based kinetic (PBK) modelling data in regulatory toxicology, the project will evaluate the applicability domain of PBK models and their input parameters, in particular the in-silico and in-vitro ADME parameters. A special focus is to better understand the performance and limitations of these methods, the current knowledge and data gaps.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine