Personalized Preclinical Care as a Basis for the Development of Personalized Therapies
The discovery and preclinical validation of new therapeutics has always involved the use of long term established cancer cell lines and animal models. The advantage here mainly lies in the standardization and detailed characterization of these models.
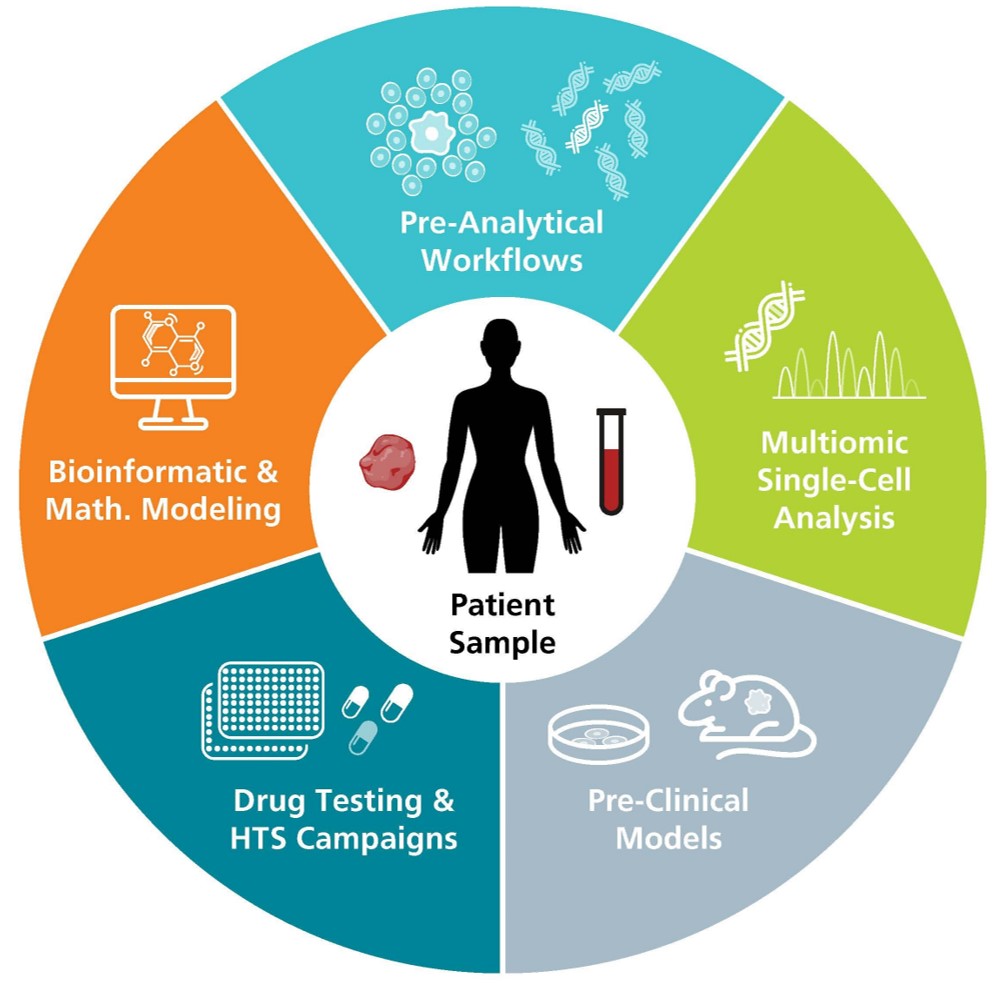
In personalized medicine, novel cancer therapies are developed on the basis of specific tumor entities. Here, it is “the right therapy for each patient” that applies, not “one drug fits all”!
This paradigm shift also necessitates rethinking the process of preclinical drug development. Here, individual model systems and biomarkers are needed to adequately represent the clinical situation as much as possible. Thus, only drugs that have also proven effective in preclinical models that reflect the disease will make their way into clinical testing.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine