Inhaled antibiotic therapy is the treatment of choice for patients with lung infections: it delivers the therapeutic agent directly to the site of infection, thus enabling higher doses with reduced systemic impact on the body. In the development of inhaled therapeutics, it is mandatory to verify whether the active ingredients are still active after nebulization and what formulation is most effective.
To this end, the Working Group on Infection, Inflammation and Allergy is developing biofilm models based on Pseudomonas aeruginosa as an example, aimed at enabling preclinical testing of novel therapeutic strategies.
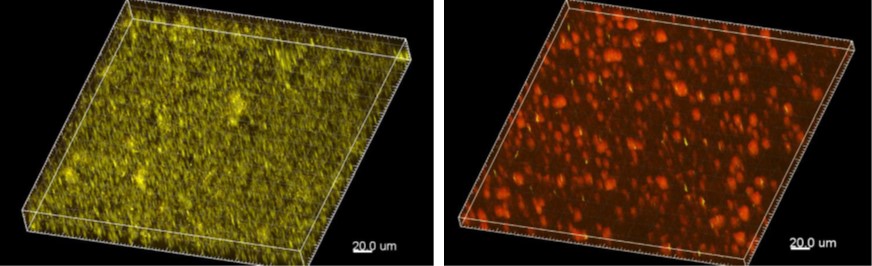
To enable reliable prediction of the efficacy of inhaled antibiotic agents against biofilm-associated infections at an early stage of drug development, Fraunhofer ITEM scientists have established an in-vitro system for exposing bacterial biofilms to inhalable antibiotics. For this purpose, biofilms of the bacterium Pseudomonas aeruginosa, a typical “problematic pathogen” in the context of lung infections, are grown in vitro and can be exposed to drug candidates. As a biofilm, these bacteria display a substantially increased resistance to antibiotics, enabling much better prediction of efficacy for biofilm-associated infections than with traditional microbiological tests.
By using an exposure system developed at Fraunhofer ITEM, therapeutic substances can be nebulized for treatment of biofilms, allowing determination of the effective dose. In the future, this system can be used for comparative testing of novel inhaled formulations or carrier systems, to enable prediction of what combination of active agents is most effective in breaking through the protective cover of biofilms and in killing the pathogens.
Our findings about how human mucus affects biofilm and its susceptibility to antibiotic treatment have been published in the “Journal of Antimicrobial Chemotherapy”.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine