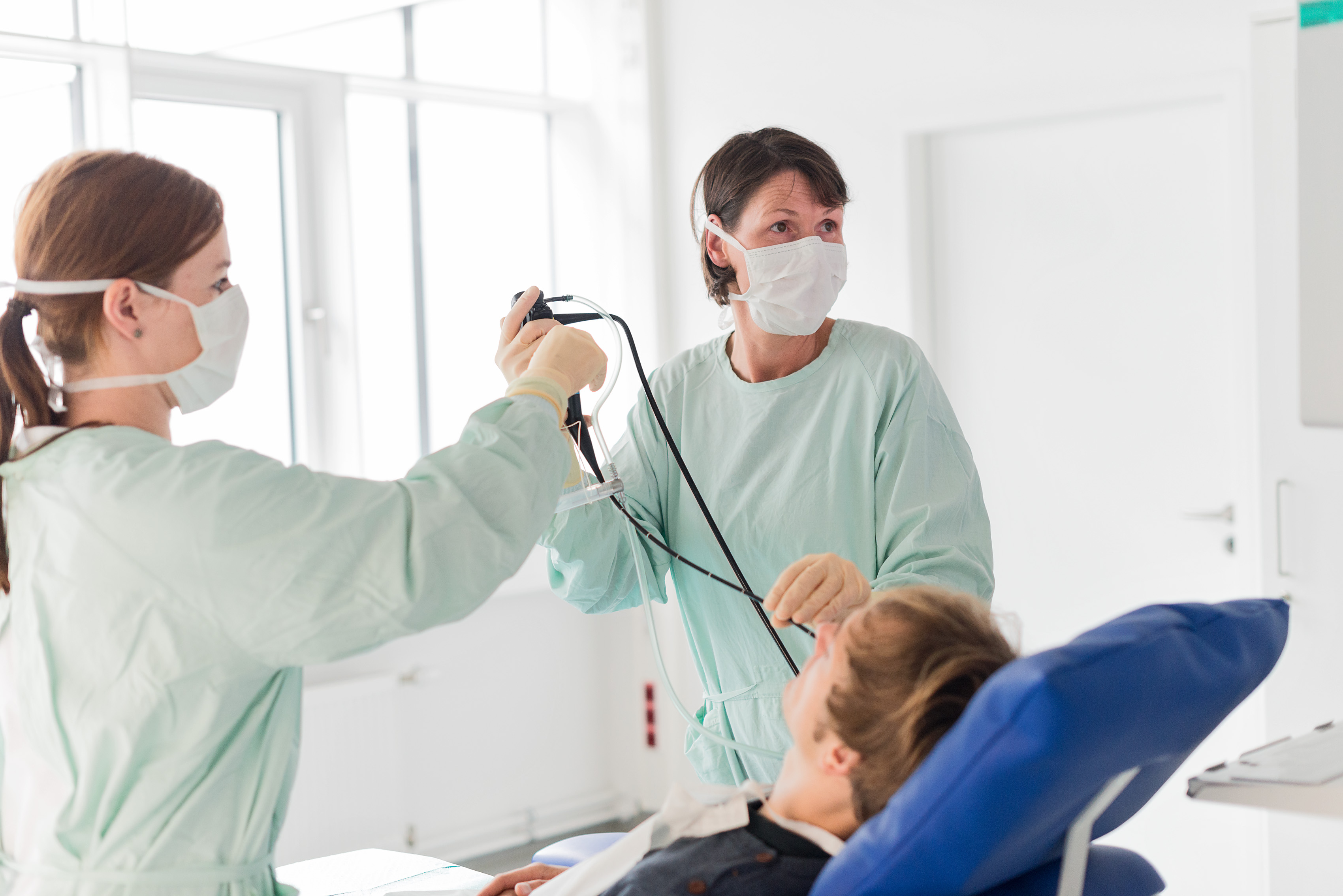
The approved COVID-19 vaccines elicit the temporary formation of antibodies and a systemic T-cell response that provide protection against a severe course of the disease, but not against infection. Most importantly, intramuscular injection of the available vaccines does not induce mucosal immunity in the respiratory tract. In cooperation with the Hannover Medical School, which is also the sponsor of this investigator-initiated clinical trial, Fraunhofer ITEM scientists are investigating the safety and efficacy of inhaled booster vaccination with the vector vaccine MVA-SARS-2-ST in a phase-I trial. This vaccine contains an attenuated strain of the vaccinia virus (Modified Vaccinia Ankara), which is related to the smallpox virus but unable to replicate. For decades already, it has been used as a platform for vaccine production and has now been developed to target the S protein of SARS-CoV-2. After successful non-clinical development and evidence of a mucosal immune response in the respiratory tract in animal models, the first study in humans involving inhaled administration of the vaccine is now being performed. In addition to assessing local and systemic safety, a focus will be on efficacy, which will be evaluated by bronchoscopy with comprehensive characterization of the humoral and cellular immune response before and after administration of the vaccine. The study is expected to be completed in mid-2023.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine