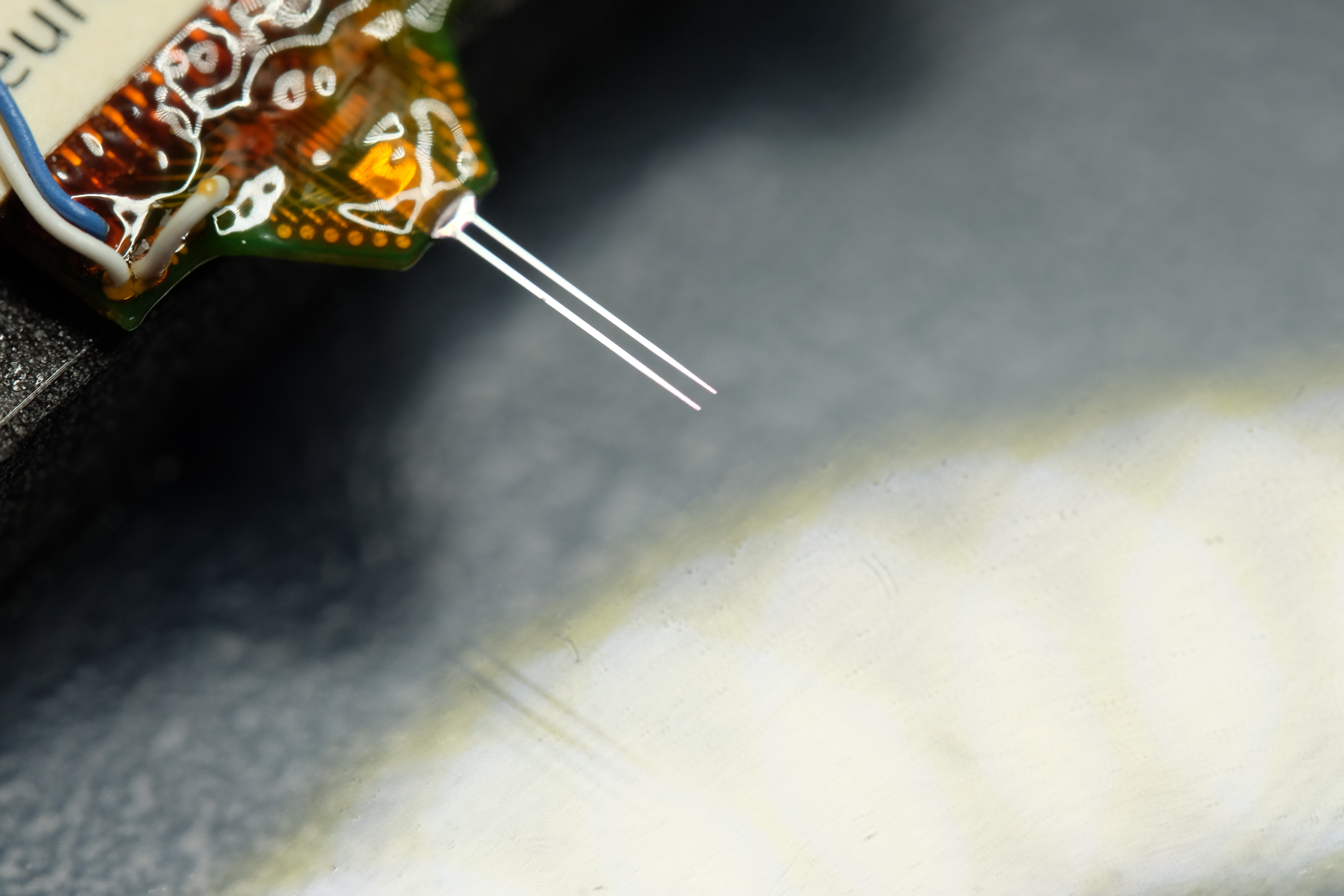
With an increasingly aging society, people wish to remain active for extended periods. Consequently, implants should be durable and stress-resistant. This also applies to neural implants, which are used, for instance, in patients with tetraplegia to significantly restore autonomy and quality of life. Extensive testing, particularly for the longevity of the implants, is thus essential; however, obtaining market approval for modern neural implants often takes decades.
Researchers at Fraunhofer ITEM focus on developing solutions that ensure the quality and durability of neural implants while accelerating their development and market entry. A well-structured methodology for developing such implants, considering the full product development cycle - covering raw materials, production, packaging, sterilization, and the surgical procedures - will enable a reduction of the average development time from 11 years and increase safety. This will help to avoid patient trauma, enhance societal acceptance, and make it more attractive for companies to engage in the field of neurotechnology.
A first step is the long-term testing of intracortical neural implants in animal models to understand different failure modes in a multi-stage testing process, including surgical techniques, implantation duration, signal quality, analysis, and histology. The goal is to overcome these challenges by developing and applying suitable quality criteria.
Through the High-Performance Center Medical and Pharmaceutical Engineering and in collaboration with scientists, engineers, manufacturers, medical professionals, patients, and regulatory experts, Fraunhofer ITEM aims to establish a dynamic platform that sets new standards in neural implant technology.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine