Fraunhofer magazine 2/2021
The race against viruses
As a consequence of climate change, tropical mosquitoes are becoming increasingly endemic to Germany, where they spread dangerous pathogens. Even local species of gnats have now become carriers. Sixty percent of infectious diseases that afflict humans originate in animals. The West Nile virus has already spread in the area around Leipzig, Halle and southern Brandenburg. Zika, dengue and others are likely to follow. It is only a matter of time until the next pandemic. What will it take to speed up vaccine production?
Zoonoses, meaning pathogens that spread from animals to humans, are becoming increasingly dangerous. The Global Virome Project, an international research initiative, estimates that 1.6 million distinct viruses are currently circulating in mammals and birds. Of these, around 700,000 are said to have the potential to infect humans. Not all of them can be spread from person to person, and not all lead to illness or even death. However, experts agree that the coronavirus will not remain a solitary case.
"If we want to effectively fight pandemics in the future, then the production of vaccines must become significantly faster,"
says Prof. Holger Ziehr, director of the Pharmaceutical Biotechnology division at the Fraunhofer Institute for Toxicology and Experimental Medicine ITEM. Working across various institutes and disciplines, he coordinates the Fraunhofer Vaccine Technologies research consortium, which was formed in response to the coronavirus pandemic. In the consortium, vaccine experts, bioprocess engineers, production engineers and packaging specialists work on making vaccines available more quickly — the importance of which became apparent during the months of the pandemic. To this end, they aim to bring vaccine candidates swiftly into clinical trials, develop and provide technology platforms, and optimize production and packaging and prepare them for mass-scale application.
The development and production of vaccines has been neglected for years all over the world. Manufacturing technology for conventional, inactivated vaccines has hardly changed since the 1940s, because any effort to do so would not have been profitable enough. “Awareness of the importance of this technology has grown significantly as a result of the coronavirus pandemic,” observes Dr. Sebastian Ulbert, who heads up research into infectious disease pathology at the Fraunhofer Institute for Cell Therapy and Immunology IZI in Leipzig. This virologist and zoonosis expert helped to set up the Fraunhofer Vaccine Technologies consortium, together with Prof. Ziehr.
Normally, it takes a number of years for a promising vaccine candidate to emerge — time that scientists simply don’t have when an epidemic or pandemic is raging. For this reason, it is crucial to identify vaccine candidates for potential pathogens before they are needed in large quantities. Dr. Ulbert and his team have already succeeded in this for the Welt Nile virus.
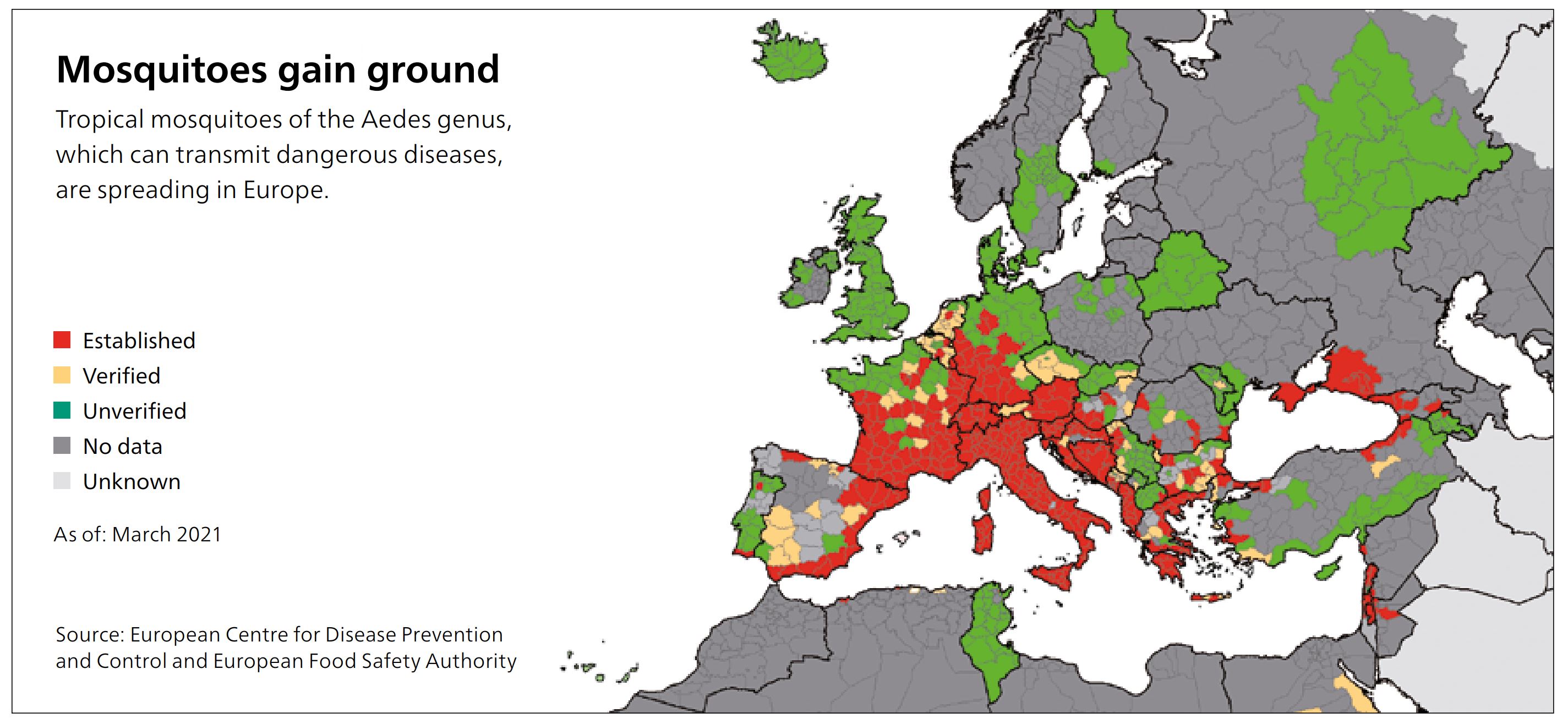
Patients often exhibit high fever, vomiting, diarrhea, exhaustion and joint pain. In certain rare cases, inflammation of the brain or meninges can also occur — as is the case with TBE, which belongs to the same virus family. In contrast to TBE, the West Nile virus is not transmitted by ticks, but by the common house mosquito. In Leipzig last summer, 11 patients with severe cases had to be treated in the hospital.
“It is important to have a vaccine ready before the virus spreads further in Germany — which it most certainly will, especially since it is much more difficult to protect yourself from mosquito bites than from tick bites,”
warns Dr. Ulbert. Now, he is searching for industry partners who can help him conduct clinical trials for his vaccine candidate. Prof. Ziehr and his team at Fraunhofer ITEM can also count a success to their names already: By shortening the conventional procedure, they succeeded in bringing an antibody agent against COVID-19 from the pre-clinical phase to the clinical phase within six months, rather than the usual two years. This so-called “passive vaccine” is intended to help the immune systems of seriously ill patients to fight off SARS-CoV-2 using externally produced antibodies. “Virus proteins for conventional vaccines could also be produced more quickly this way, and without any problems,” says Prof. Ziehr. So how did they do it? Prof. Ziehr and his team did not reproduce the clone. This is the cell that most effectively produces antibodies, but is difficult to identify. Rather, they reproduced an entire cell pool, which accomplishes the same task — although the individual cells are perhaps less productive.
“In a previous project, we had established that the cells which were derived from the clone were no longer entirely identical after many divisions. Obviously, mistakes occurred in the process,” explains Prof. Ziehr. This raised the question: Is finding the clone even worth the effort? Especially during a pandemic, when quick solutions are imperative? They dared to experiment, putting the initial suspension of around 5,000,000 cells under evolutionary pressure, which allowed them to find those that could produce the antibody. “The result proved us right. The cells produce large quantities of pharmaceutical-grade antibodies, which are already being used to treat patients."
”Even the most effective vaccine, however, is useless if it cannot be bottled!”
This is another significant weakness that the coronavirus pandemic brought to light. “In Europe alone, we need hundreds of millions of glass vials for injection bottles, but they simply don’t exist. The production capacities are limited,” explains Prof. Ziehr. Consequently, researchers at the Fraunhofer Institute for Surface Engineering and Thin Films IST and at Fraunhofer IPK are developing alternatives made of plastic, which can be produced more quickly and much more cost-effectively through injection molding. Moreover, the polymer ampules are shatterproof, lighter and therefore more suitable for transport. But there is a problem: The plastic must not react with the vaccine and render it unusable. A protective coating could be the solution.
“We are pursuing a number of approaches so that in the future, we can better keep pace with new viruses. The fact that we have built connections with each other in the research consortium is a big advantage here. It means that, for example, all the stakeholders can be brought in even in the pre-clinical phase, and requirements for mass producing the vaccine are taken into account. Processes take place simultaneously, not one after the other,” explains Prof. Ziehr. “That saves time, reduces costs and leads to the best result.”
Article by Dr. Sonja Endres, published in Fraunhofer magazine 2/2021
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine