Comprehensive database to facilitate the assessment process
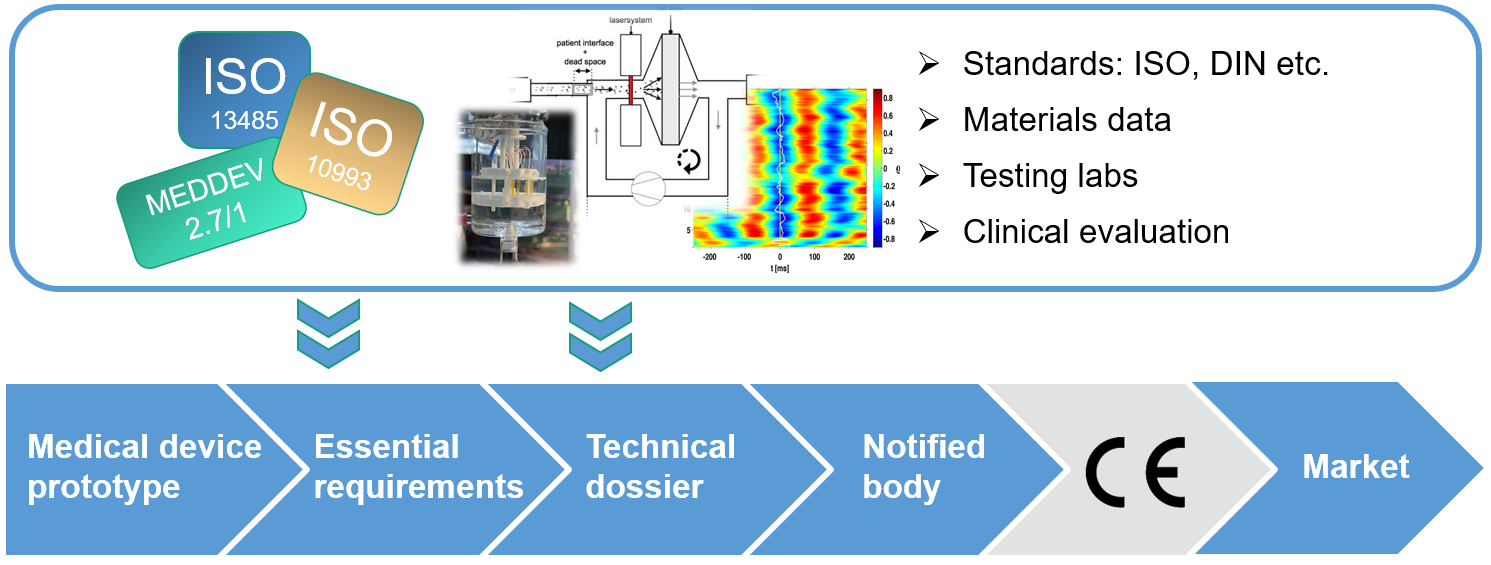
MDOT is addressing medical device manufacturers’ need for support with the obligatory conformity assessment, which has been exacerbated by the EU regulation 2017/745 (Medical Device Regulation, MDR for short). Under this regulation, coming into force at the end of May 2020 and replacing the Medical Device Directive (MDD), all existing medical devices need to undergo reassessment of their risk class, require more comprehensive documentation and increased clinical testing. To reduce the burden on medical device manufacturers, the consortium is developing a platform aimed at simplifying the process by establishing a database that will include data on regulatory affairs and testing up to clinical evaluation and clinical studies.
To demonstrate usability of the platform, it is addressing three technologies as a starting point: inhalers for pre- and early-term neonates, 3D-printed neural implants, and coatings for orthopedic prostheses that reduce the wear of particles in the patient’s tissue. Test beds for these technologies are being developed and upgraded to ensure safety and long-term stability of devices and materials – aspects particularly important for implants. Future extension of the platform towards other medical device sectors is planned.

 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine