Increasing patients’ access to high-risk medical devices
Aiming to support European manufacturers of medical devices the global competition, the EU has initiated the project TBMED – An Open-Innovation Test Bed for the Development of High-Risk Medical Devices. Together with 12 project partners from Spain, France, Ireland and Germany, Fraunhofer ITEM is actively participating in the development of a test bed for medical devices of risk classifications IIb and III. The project will receive 8.5 million euros funding under the European Union’s Horizon 2020 Framework Program and will run until the end of February 2023.
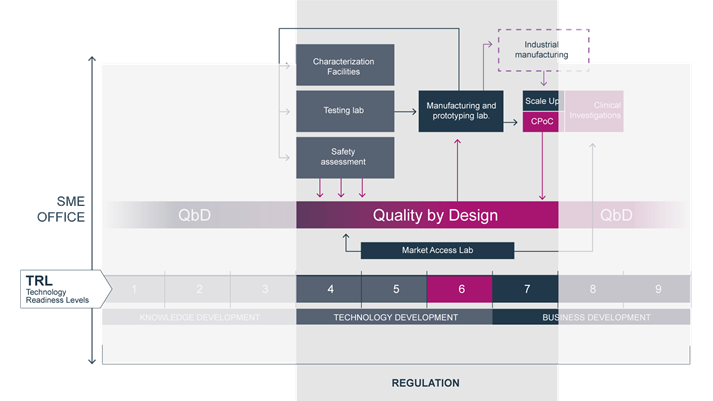
TBMED is an EU-funded collaborative research project that aims to increase patients’ access to high-risk medical devices by helping SMEs minimize time to market and reimbursement process time, thus optimizing the process of transforming a prototype into a valuable innovative medical device (MD).
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine