Challenges in the care of preterm infants
In today’s medical landscape, the care of preterm infants faces significant challenges, particularly concerning respiratory diseases and lung injuries. Global statistics indicate that approximately 14.8 million babies are born prematurely each year, with around 34% suffering from severe respiratory conditions. This issue is particularly critical for extremely premature infants (born before the 23rd week of gestation), with studies from the U.S. reporting mortality rates as high as 43%.
It is well established that early systemic administration of corticosteroids (before the 7th postnatal day) can reduce the incidence of bronchopulmonary dysplasia (BPD) and mortality. However, this approach is associated with an increased risk of adverse effects such as hyperglycemia, hypertension, gastrointestinal bleeding, gastrointestinal perforations, and growth impairments. The link between early (<7 days of life) systemic corticosteroid administration and negative effects on neurological development has ultimately led to a reduction in the use of corticosteroids in ventilated preterm infants.
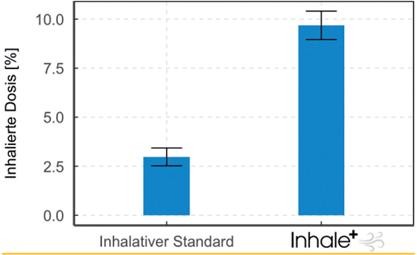
An alternative to systemic administration is early, localized delivery via inhalation directly into the lungs. Evidence suggests that when effectively administered, local inhalation can successfully reduce the incidence of BPD and mortality without the associated systemic side effects.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine