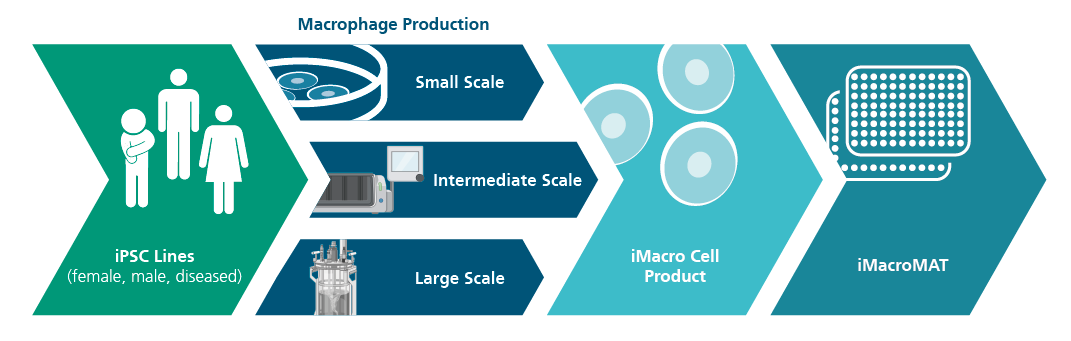
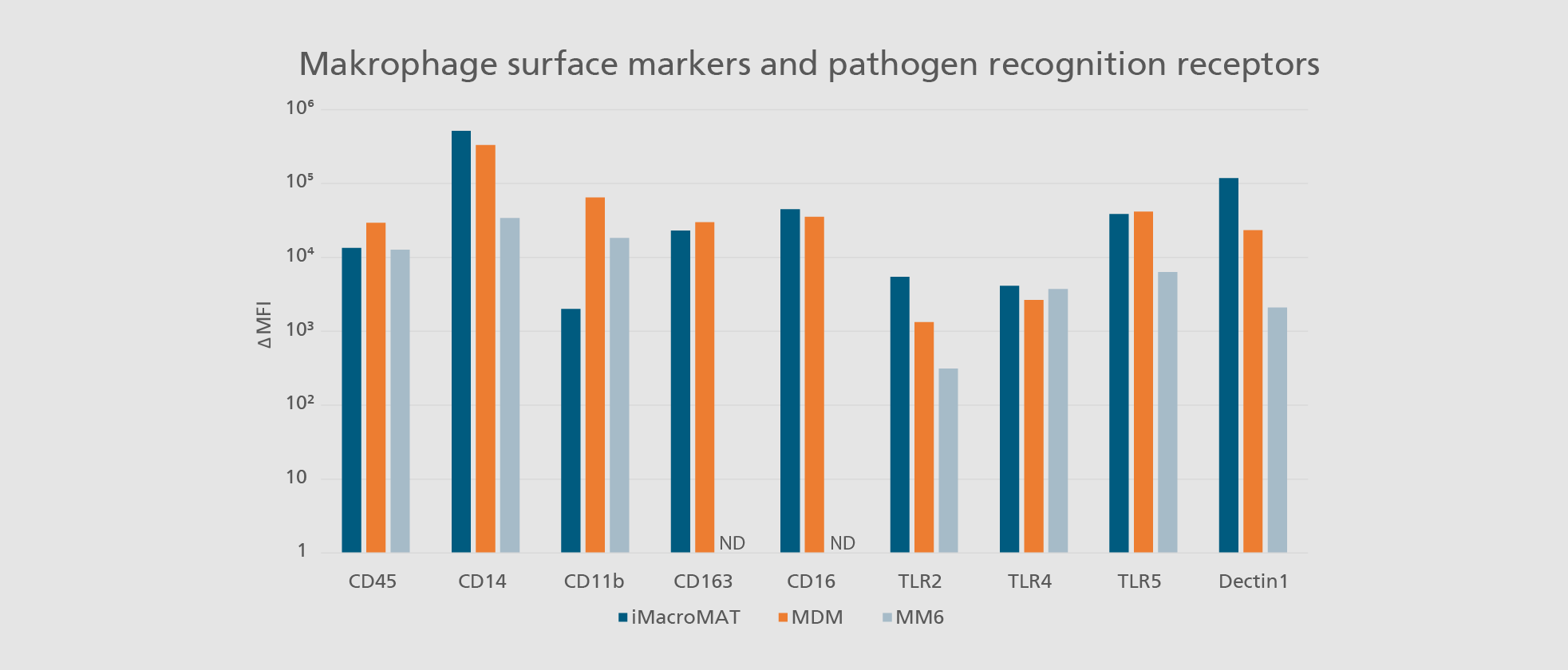
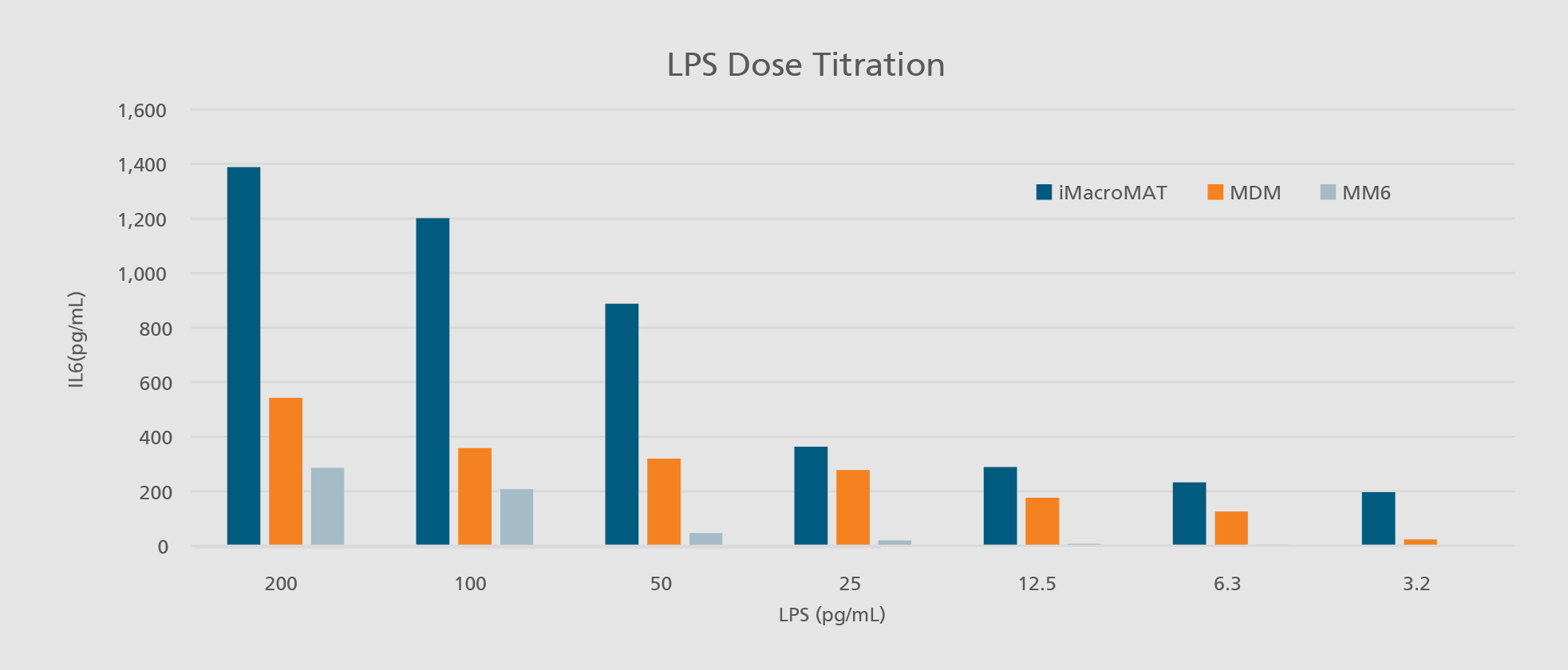
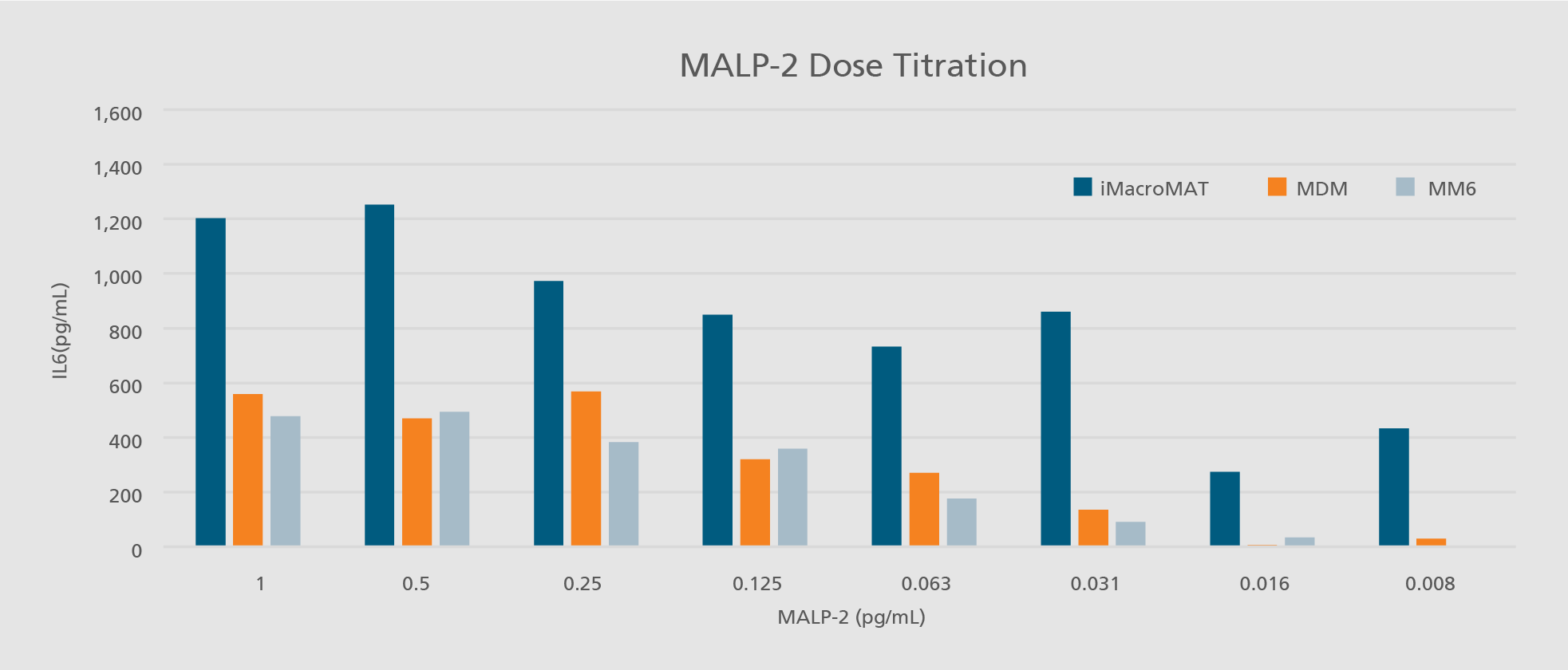
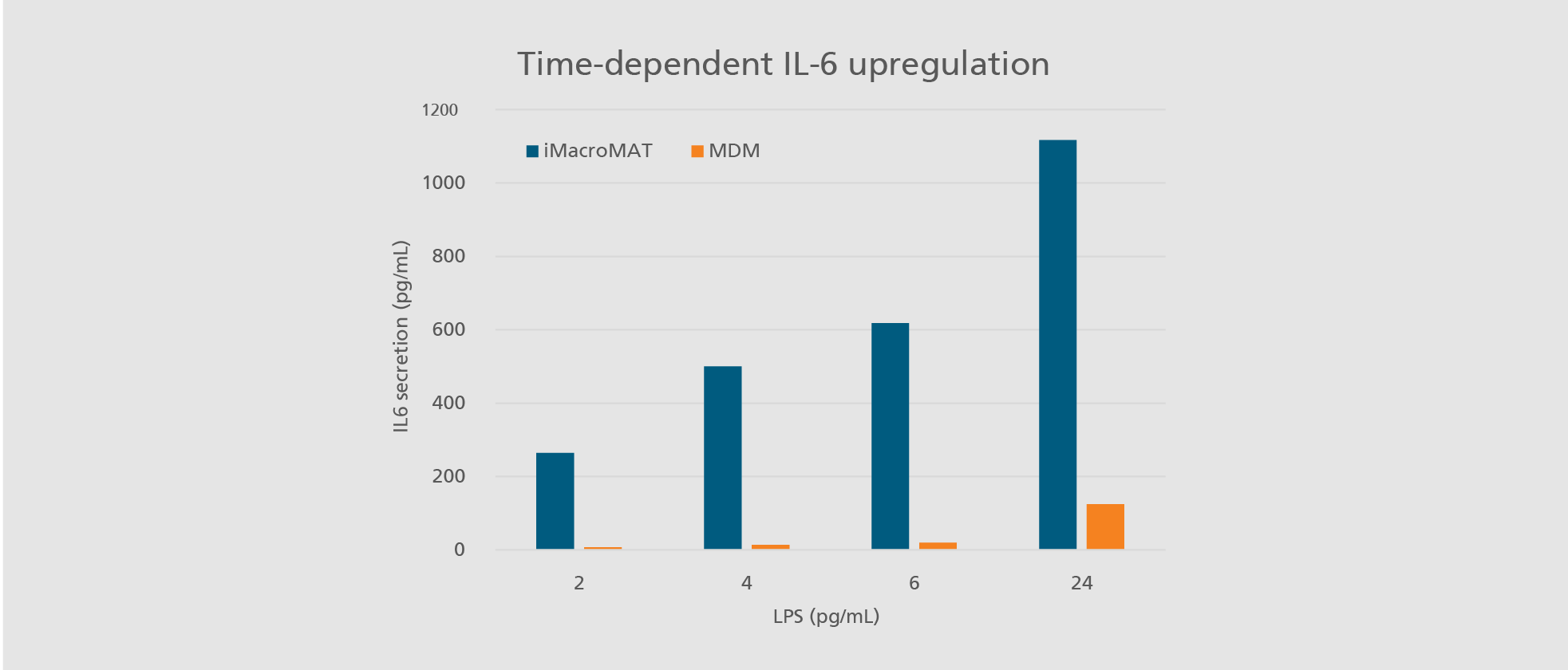
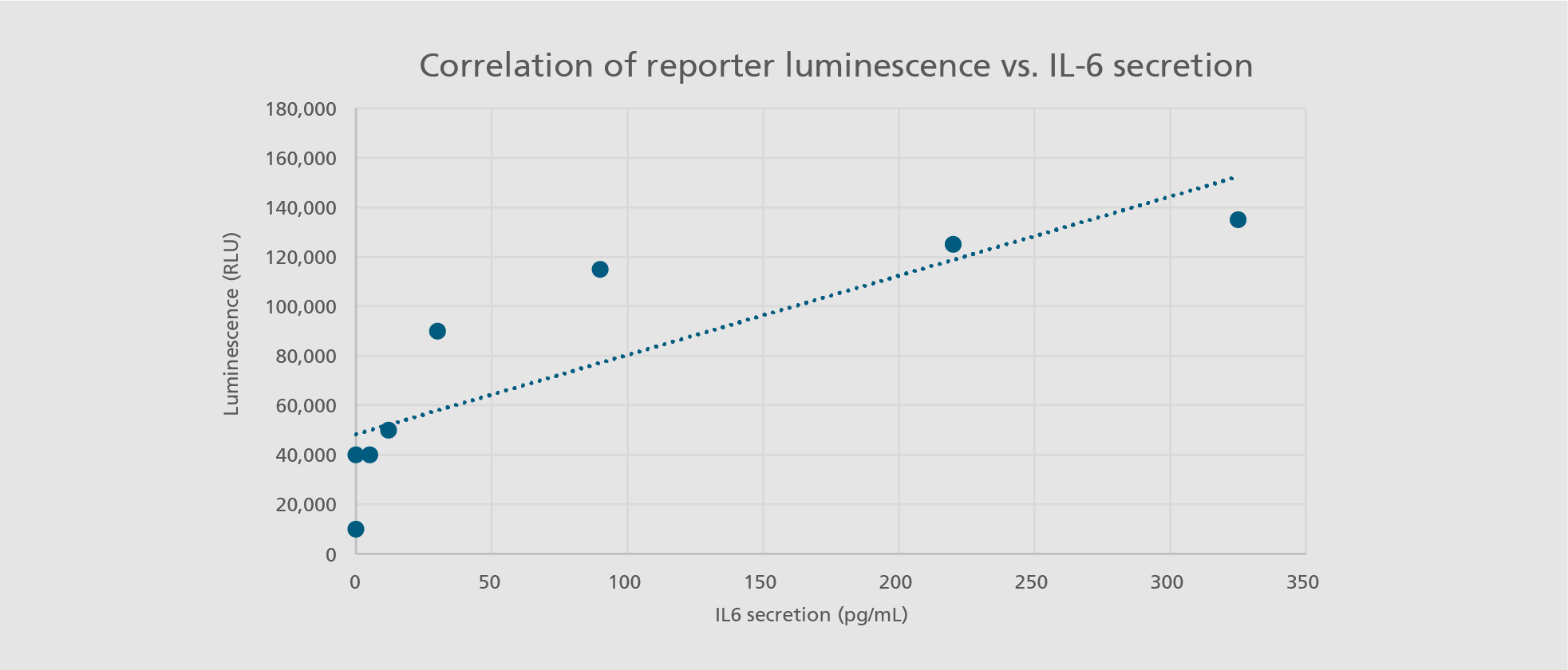
Abdin SM, Mansel F, Hashtchin AR, Ackermann M, Hansen G, Becker B, Kick B, Pham N, Dietz H, Schaniel C, Martin U, Spreitzer I, Lachmann N. "Sensor macrophages derived from human induced pluripotent stem cells to assess pyrogenic contaminations in parenteral drugs". Biofabrication. 2024 May 17;16(3). doi: 10.1088/1758-5090/ad4744.
Desai O, Köster M, Kloos D, Lachmann N, Hauser H, Poortinga A, Wirth D. "Ultrasound-triggered drug release in vivo from antibubble-loaded macrophages". J Control Release. 2024 Dec 19:378:365-376. doi: 10.1016/j.jconrel.2024.12.007.
Jacobsen C, Plückebaum N, Ssebyatika G, Beyer S, Mendes-Monteiro L, Wang J, Kropp KA, González-Motos V, Steinbrück L, Ritter B, Rodríguez-González C, Böning H, Nikolouli E, Kinchington PR, Lachmann N, Depledge DP, Krey T, Viejo-Borbolla A. "Viral modulation of type II interferon increases T cell adhesion and virus spread". Nature Communications. 2024 Jun 22;15(1):5318. doi: 10.1038/s41467-024-49657-4.
Paasch D, Lachmann N, "CAR macrophages tuning the immune symphony of anti-cancer therapies". Cell Stem Cell. 2024 Jun 6;31(6):791-793. doi: 10.1016/j.stem.2024.05.006.
Abdin SM, Paasch D, Lachmann N. "CAR macrophages on a fast track to solid tumor therapy". Nat Immunol. 2024 Jan;25(1):11-12. doi: 10.1038/s41590-023-01696-7.
Abdin SM, Paasch D, Kloos A, Oliveira MC, Jang MS, Ackermann M, Stamopoulou A, Mroch PJ, Falk CS, von Kaisenberg CS, Schambach A, Heuser M, Moritz T, Hansen G, Morgan M, Lachmann N. "Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia". J Immunother Cancer. 2023 Dec 22;11(12):e007705. doi: 10.1136/jitc-2023-007705.
Malainou C, Abdin SM, Lachmann N, Matt U, Herold S "Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting". J Clin Invest. 2023 Oct 2;133(19):e170501
Forde AJ, Kolter J, Zwicky P, Baasch S, Lohrmann F, Eckert M, Gres V, Lagies S, Gorka O, Rambold AS, Buescher JM, Kammerer B, Lachmann N, Prinz M, Groß O, Pearce EJ, Becher B, Henneke P., "Metabolic rewiring tunes dermal macrophages in staphylococcal skin infection". Science Immunol. 2023 Aug 18;8(86):eadg3517. doi: 10.1126/sciimmunol.adg3517. Epub 2023 Aug 11.
Ackermann M, Rafiei Hashtchin A, Manstein F, Carvalho Oliveira M, Kempf H, Zweigerdt R, Lachmann N. "Continuous human iPSC-macrophage mass production by suspension culture in stirred tank bioreactors". Nat Protocols. 2022 Feb;17(2):513-539. doi: 10.1038/s41596-021-00654-7
Ackermann M, Kempf H, Hetzel M, Hesse C, Hashtchin AR, Brinkert K, Schott JW, Haake K, Kühnel MP, Glage S, Figueiredo C, Jonigk D, Sewald K, Schambach A, Wronski S, Moritz T, Martin U, Zweigerdt R, Munder A, Lachmann N "Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections". Nature Communications. 2018 Nov 30;9(1):5088. doi: 10.1038/s41467-018-07570-7.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine