Platform for Ex-Vivo Drug Response Assays (PEDRA)

The Fraunhofer ITEM has long-standing experience in the assessment of drug safety and efficacy. With our special expertise in the fields of preclinical and clinical drug testing we are dedicated to supporting our clients in the development of novel targeted- and immuno-oncological therapies. By utilizing clinical samples, we generate molecular and functional preclinical data for your projects, whereas our in-house GDPR-compliant data management and bioinformatics allow custom-fit data analysis and supports you during the course of project. Our comprehensive portfolio enables us to provide fully flexible, scalable, and tailor-made R&D solutions as well as regulatory support by our experts with a track-record in communicating with the respective authorities to facilitate the market readiness of your applications products.
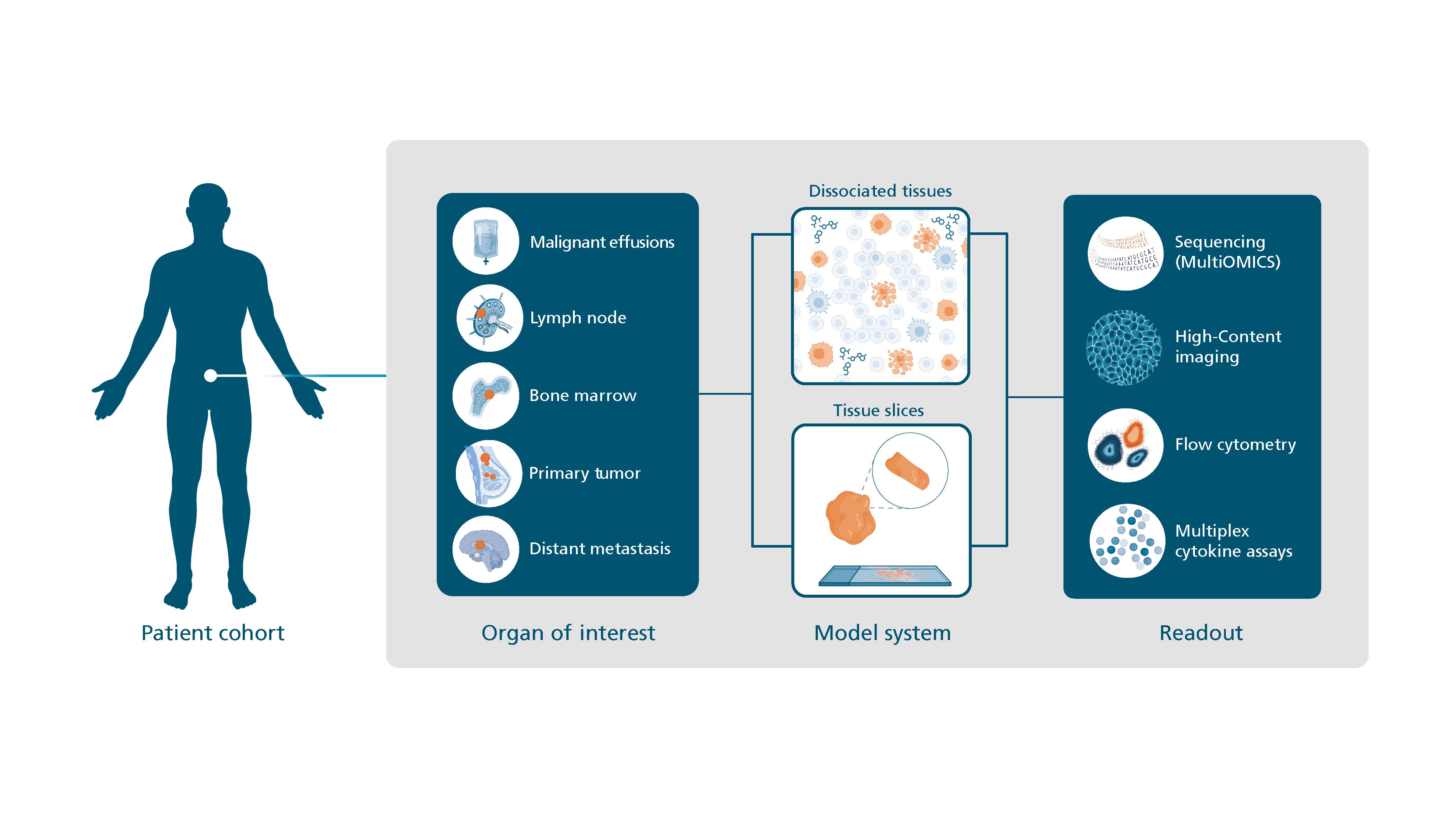
We have developed a Platform for Ex-Vivo Drug Response Assays (PEDRA) that mirrors the clinical patient situation in vitro by using disease-specific models generated from clinical samples, recruited individually for each project. PEDRA comprises several ex-vivo assays of variable cellular complexity. In combination with state-of-the-art readouts drug safety and efficacy can be assessed for a broad range of anticancer treatments. Because PEDRA preserves the original cellular composition of the organ of interest, it is ideal for testing novel IO therapies incl. CPI and ADC.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine