The important role of inhaled aerosol therapies in medicine has been acknowledged for several decades. Besides the traditional applications for local treatment of obstructive pulmonary diseases, the use of inhaled aerosols for treating lung infections and for vaccination has been gaining increasing interest. Furthermore, systemic administration of active pharmaceutical ingredients by inhalation is getting more and more important. Compared with other routes of administration (intake of tablets), however, drug dosing is challenging for the inhaled aerosol route, as it depends on aerosol and breathing parameters and the patient’s current health status.
Because of the important role of aerosol therapies, a broad range of stationary and mobile devices for inhaled administration of pharmaceutical agents via the solid or liquid phase are already available, however, substantial gaps still remain as well. The amount of active pharmaceutical ingredient that can be administered by inhalation within an acceptable time span is limited, in particular if the drug product is a dry powder; for portable inhalers it is < 50 mg per single dose. Due to their insolubility in water, numerous pharmaceuticals are, as yet, not available for continuous inhalation treatment and for high dosage. Furthermore, the use of powder inhalers is not yet state of the art in patients receiving ventilation support, neither in adults nor in children or infants. This means that whole groups of patients are excluded from treatment with certain drugs, or else administration of the drug is associated with considerable risks. The aim of the research activities at Fraunhofer ITEM is to close the existing gaps and minimize risks.
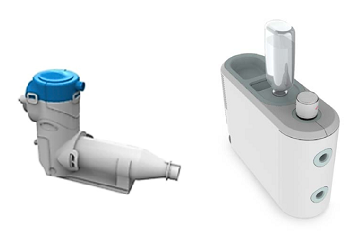
A technology allowing administration of high doses of dry-powder medication, as needed, for example, for inhalation treatment of lung infections or surfactant deficiency, will substantially enhance the application spectrum of surfactant formulations and other non-soluble drug substances or even enable their administration. This holds true for both mechanically ventilated and spontaneously breathing patients.
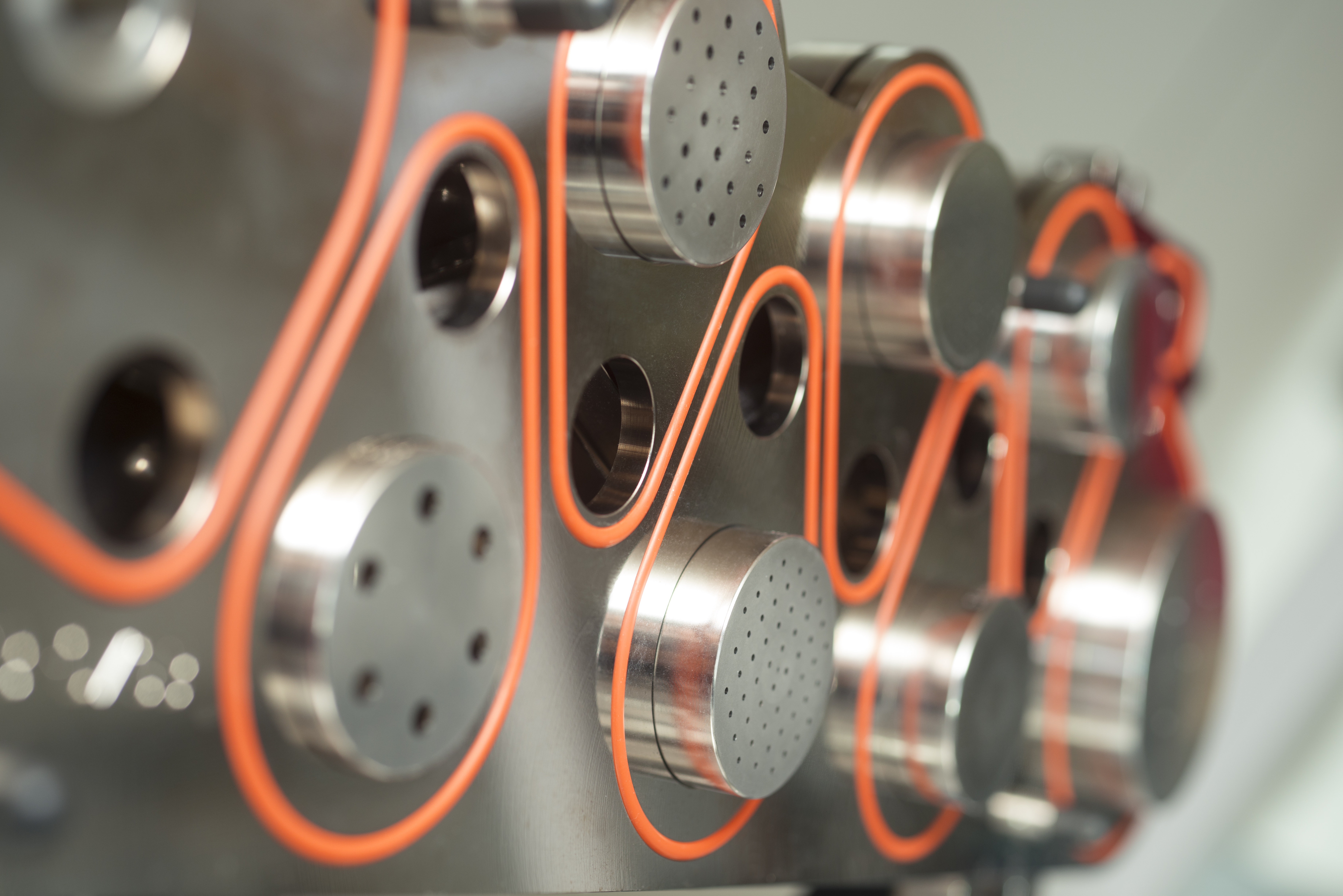
There is, in fact, a substantial medical need for inhaled administration of aerosolized drugs in children, infants, and preterm neonates. Another need that has not yet been sufficiently met is local antibiotic treatment of lung infections, in particular in patients receiving ventilation support. In order to close this gap, Fraunhofer ITEM scientists are working to develop dry-powder administration systems for adults and preterm neonates, including appropriate test methods for such devices. This work is based on more than 30 years of experience in the areas of aerosol technology and aerosol administration to humans. The measurement methods we use include both traditional aerosol measurement technology and special measurement methods for inhaler characterization. Methods of fluid dynamics and thermodynamics complement our instrumentarium.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine