Animal testing is essential for achieving progress in biological and medical research. It provides insights that can then be used to develop new medicines and treatments, and to assess health risks. These findings are so beneficial to humans, but come at a cost to the animals.
At Fraunhofer ITEM, we test the efficacy and safety of drugs, chemicals and medical devices. We perform these pharmacological, toxicological and safety pharmacology studies on rodents. Our studies are conducted in accordance with the international approval regulations issued by the OECD and the EMA. In 1991, the institute was granted GLP certification pursuant to the Chemicals Act or Directive 2004/9/EC.
It goes without saying that, in our research work, we treat all animals humanely and observe strict hygiene conditions, and that all tests are conducted responsibly. Our veterinarians and animal keepers, as well as our animal welfare officers, are involved in the planning and execution of the tests.
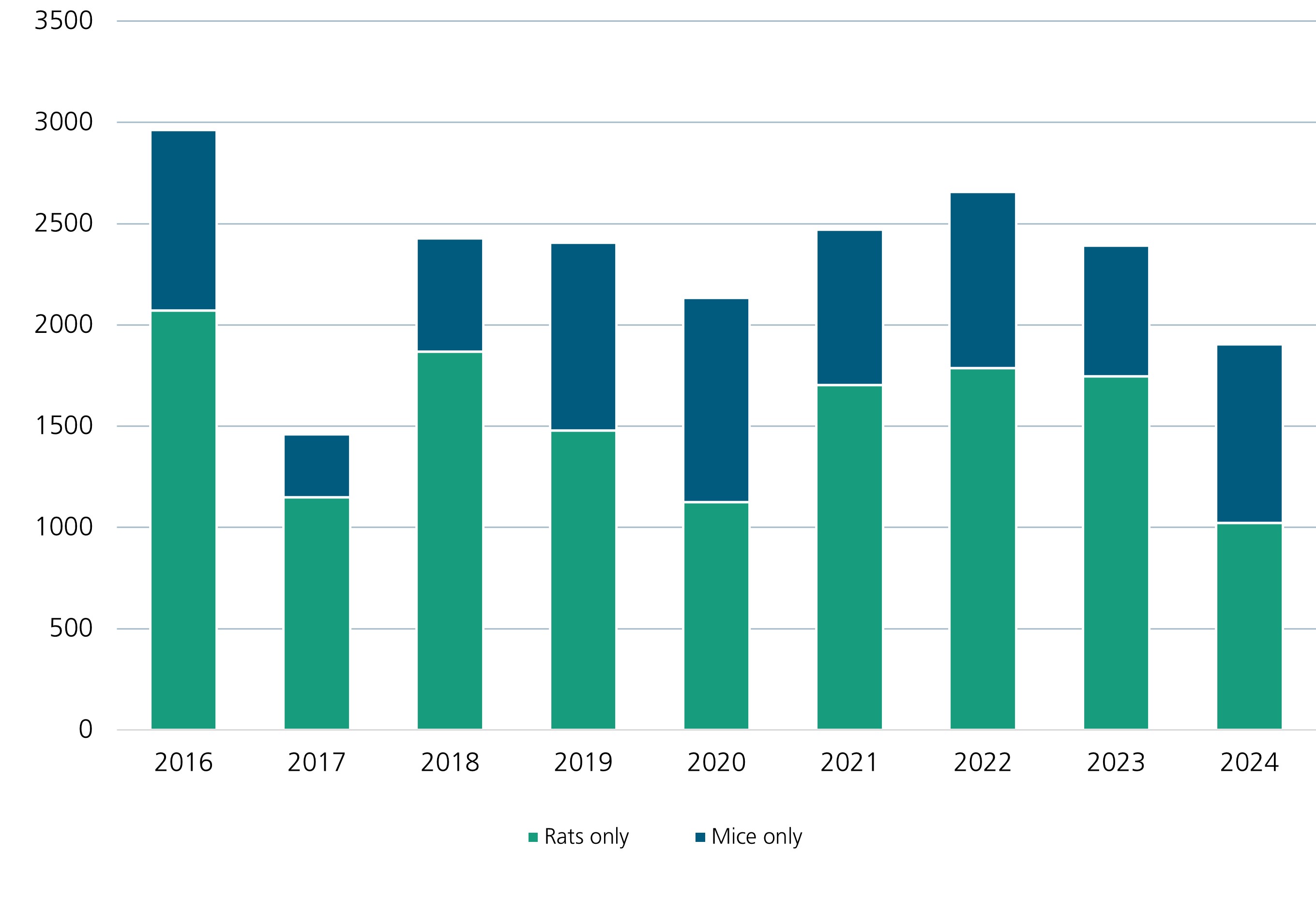
For many years, we have been working to minimize animal testing, replace it with alternative processes, and develop and establish new alternative methods. In recent years, an average of 2,000 to 2,500 mice and rats have been used for animal studies at Fraunhofer ITEM. For comparison, here is a detailed record of the numbers of animals used in research in Germany.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine