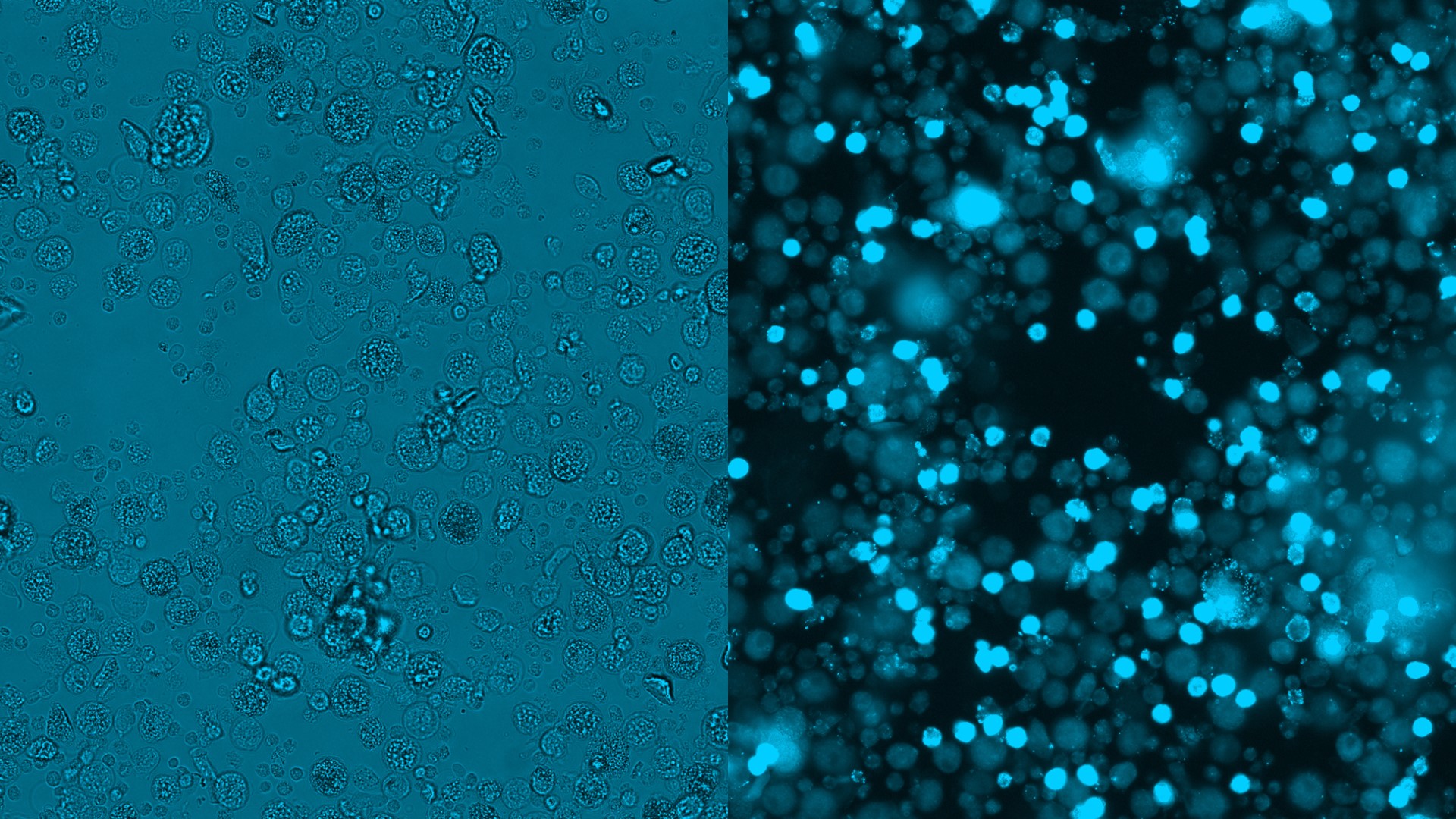
Chip cytometry is opening up new possibilities in clinical research
Chip cytometry is a novel tool that combines the analytical capabilities of cell differentiation by microscopy and flow cytometry. The cells are transferred to specific microfluidic chambers, referred to as chips, and fixed. This allows a temporal separation of sample preparation and cytometric measurement without compromising sample quality. By means of fluorescence-stained biomarkers, these chips can be used to investigate cell morphology, expression of surface markers, and intracellular functions. A special advantage is that the measurement does not cause the cells to be lost and that they can thus be re-analyzed repeatedly for comprehensive immunological and functional characterizations. Depending on the cell population, analyses at the single-cell level and also storage of the chips are possible over prolonged periods of time. Furthermore, the method facilitates sample transportation. This is particularly important for sample analyses in multi-center trials, when samples from different clinical sites, which are usually located far away from each other, are to be centrally analyzed in a core facility.
Differential cell count by microscopy and flow cytometry are less appropriate methods of analysis in multi-center clinical trials
Chip cytometry excels over other methods of analysis that are commonly used: it combines direct optical analysis of cells with the possibility to repeatedly label single cells with antibodies and analyze them, while preserving the sample material after the measurement. Among the other methods of analysis are differential cell count by microscopy and flow cytometry. The first one is fast, simple, inexpensive, and available in many clinical laboratories. It allows adequate analysis of changes in the cellular inflammatory response of the major cell populations. A differentiation of monocytes and small macrophages, however, is difficult due to their overlapping morphological features. Moreover, analysis of rare cell populations is inaccurate and the amount of information that can be obtained is limited.
Flow cytometry, in contrast, allows more detailed cellular characterization and is able to distinguish between monocytes and macrophages. In addition, it detects rarer cell populations more accurately. However, this technology requires comprehensive expertise and professional equipment. In multi-center clinical trials, lack of instrumentation, extensive requirements for technical harmonization or known inter‐laboratory variability hamper the use of flow cytometry. Alternatively, samples could be measured and analyzed in a central laboratory, but this is difficult because preservation, storage, and shipment of cells can affect cell viability and the activation status of cells and thus sample quality.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine