Paradigm shift moving towards mechanistic risk assessment
The LRI C9 project ZET-O-MAP aims to develop better tools for the identification of teratogenic compounds. One of the most promising assays is the zebrafish embryo teratogenicity assay (ZETA).
In human risk assessment the evaluation of developmental toxicity or teratogenicity requires the testing of rodents (preferably rats) and non-rodents (preferably rabbits) as described in the OECD 414 guideline. Currently, human safety assessment is undergoing a paradigm shift moving towards mechanistic risk assessment and there is a high demand to replace, reduce or refine animal tests where possible for ethical and economic reasons.
Approach of ZET-O-MAP
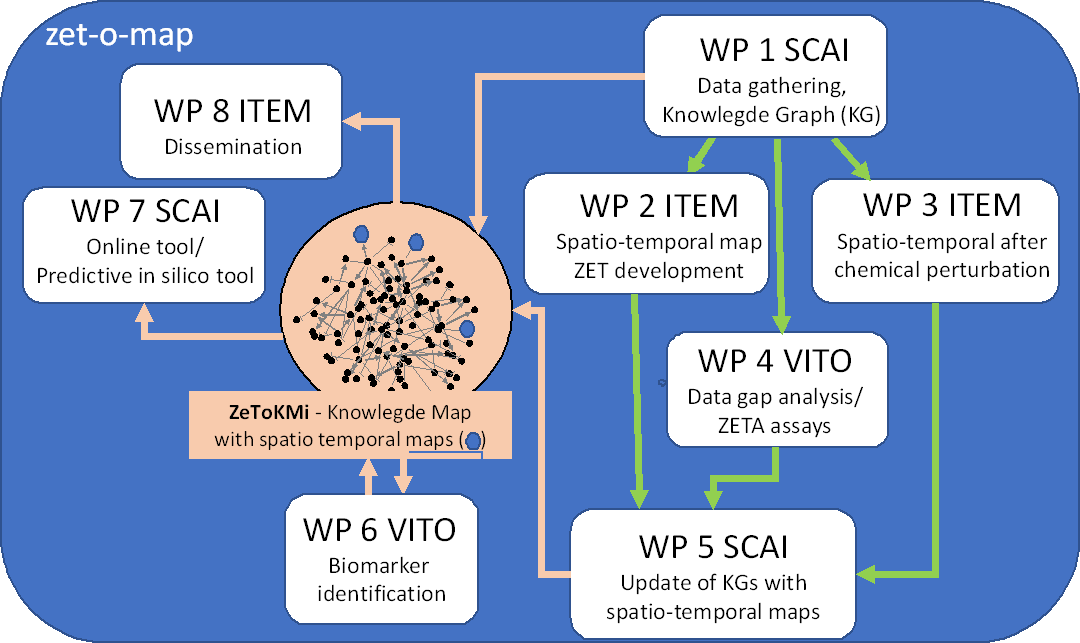
To promote the acceptance of the ZETA for regulatory purposes, a better mechanistic understanding of the morphogenesis in zebrafish embryos is needed. In order to map developmental outcome pathways, early molecular changes and morphological changes need to be linked. To identify adverse outcome pathways (AOPs), causal links with chemical exposure should be investigated and mapped.
ZET-O-MAP will develop a knowledge map covering the whole developmental process from fertilization to 120 hpf using an innovative data mining/network approach which comprises a network of early molecular changes (MC) and adverse outcomes (AOs) scored as teratogenic changes (TC) in ZETA.
In order to build this knowledge map, transcriptome data will be analyzed and linked to the adverse outcomes (AOs), indicating the development over time. In this mechanism-driven approach, ZET-O-MAP will identify principal molecular drivers of (dys)morphogenesis by analyzing time-resolved ZETA transcriptome data and link these to adverse outcomes, e.g. induced by certain teratogenic compounds.
 Fraunhofer Institute for Toxicology and Experimental Medicine
Fraunhofer Institute for Toxicology and Experimental Medicine